More women than ever before are choosing to augment their breasts with implants.
This increase is also causing a rise in another surgical procedure: breast implant removal.

While many of the women that undergo this procedure do so to correct an implant complication or because they have changed their aesthetic preference, others do so for a third reason: the fear of breast implant-associated diseases.
Even though breast implants are safe and severe complications are rare, some women decide that their benefits are not worth the risk.
What Is Breast Implant Illness (BII)?
BII acknowledgment has gained momentum in recent years due to an increase in information available on social media.
Unfortunately, while this information is easily accessible, it is not always accurate.
Breast implant illness is not an actual medical diagnosis, and there is no proof that it exists.
Breast implant illness is the name coined by a select group of women who have experienced a host of bizarre side effects that are similar to an autoimmune disease.
While the presence of breast implants is a shared link between these women, no scientific data has been able to find a connection.
What Are the Symptoms of BII?
Women who feel they are experiencing breast implant illness develop the following symptoms:
- Joint pain
- Muscle soreness
- Headaches
- Fatigue
- Chest pain
- Hair loss
- Skin rashes
- Anxiety
- Depression
- Chills
- Photosensitivity
What Are Your Treatment Options for BII?
As breast implant illness is not a recognized illness, there are no set treatment rules. That said, the symptoms are still real and should never be ignored.
Some women do experience a reduction of symptoms following the removal of their implants, but this may not be the case for everyone.
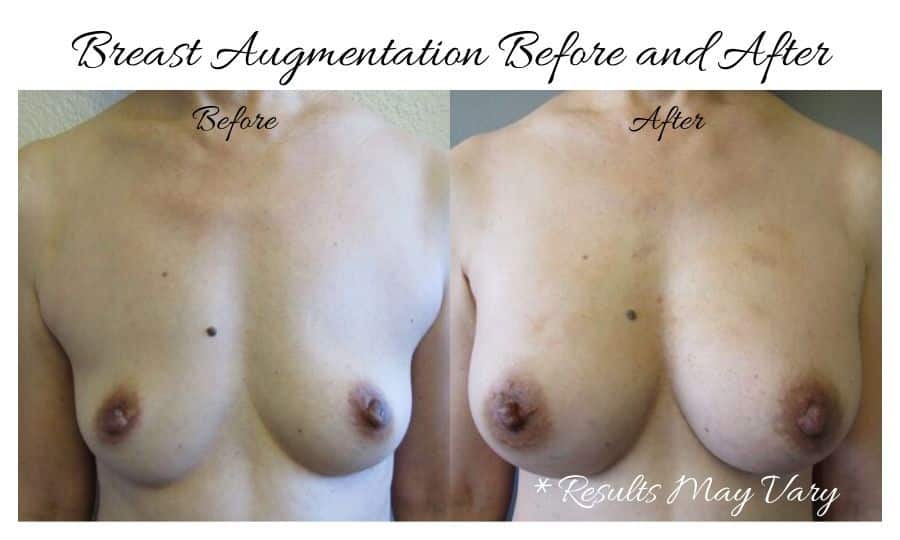
What Is Breast Implant-Associated Anaplastic Large-Cell Lymphoma (BIA-ALCL)?
BIA-ALCL is a rare cancer of the immune system that develops in scar tissue. It is not breast cancer.
A capsule of scar tissue is created around the implant after your breast augmentation. It is believed that cancer can develop in the cells of this tissue.
BIA-ALCL has been seen in patients with both silicone and saline implants, which means that the filling material has little to nothing to do with the development of the disease.
Instead, BIA-ALCL is linked to the texture of the implants. Breast implants are available in smooth and rough surfaces. Rough textures keep the implant in its place in the breast pocket.
This is important for anatomically shaped implants as they must remain in the correct direction for aesthetically pleasing results.
As of now, the only cases of BIA-ALCL have been found with rough-textured implants. These implants often cause more inflammation than smooth implants do, and this may be partially to blame.
What Are the Symptoms of BIA-ALCL?
Women suffering from breast implant-associated anaplastic large-cell lymphoma experience some of the following:
- Breast enlargement
- Pain
- Breast asymmetry
- Lumps
- Rashes
- Hardening of the breast
- Fluid accumulation
What Are Your Treatment Options for BIA-ALCL?
While BIA-ALCL is cancer, it is very treatable when discovered early and may not require chemotherapy or radiation.
Your treatment will begin with the removal of the implant and the scar tissue capsule. The removal of these is often sufficient for women whose cancer has not spread outside of the tissue.
For women whose cancer has spread to other regions of the body, additional treatment with chemotherapy, radiation, or stem cell transplant therapy may be required.
The development of this disease is extremely rare. As of now, the FDA and many physicians only recommend removing your textured implants if you are experiencing any complications.
Find Out More
To find out more about your risk and the safety of implants, contact Dr. Boll by calling (480) 833-5200 or by filling out our online contact form.
Dr. Boll sees patients from Tempe, Phoenix, and Scottsdale, Arizona.
